Research
Chemists develop a fundamentally new mode of adsorption
October 22, 2021
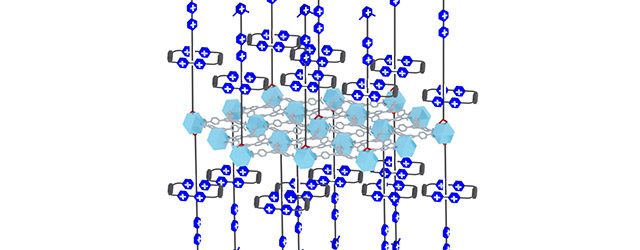
Rings mechanisorbed onto a solid surface.
Method holds promise for numerous applications, including energy storage
A research team, led by Northwestern University chemists, has made a breakthrough in surface science by introducing a new active mechanism of adsorption. Such adsorption-based phenomena, in which molecules are attracted onto a solid surface, are essential for today’s catalysts, energy storage and environmental remediation.
The research demonstrates how artificial molecular machines — wholly synthetic molecular components that produce machine-like movements — grafted on surfaces can be used to recruit molecules actively onto these surfaces at very high concentrations, thereby storing significant amounts of energy.
The new adsorption mechanism, called mechanisorption, results from non-equilibrium pumping to form mechanical bonds between the adsorbent (the surface) and the adsorbate (the molecules). Details of the study, titled “Active mechanisorption driven by pumping cassettes,” was published online today (Oct. 21) in the journal Science.
The mechanism uses redox (i.e., reduction followed by oxidation) and acid-base chemistry to adsorb and desorb a fleet of rings precisely onto and off the surface of a solid-state two-dimensional metal-organic framework (MOF). In the study, the molecules brought to the surface were rings, but it is anticipated that the approach can be generalized to include many other molecules by functionalizing the rings for a start.
“The importance of this piece of research lies in the fact that it is the first major fundamental advance in surface chemistry since physisorption and chemisorption — both equilibrium-based phenomena — were the order of the day back in the 1930s,” said Northwestern’s Sir Fraser Stoddart, who received the 2016 Nobel Prize in Chemistry for his work involving the design and synthesis of molecular machines.
Stoddart, Board of Trustees Professor of Chemistry at the Weinberg College of Arts and Sciences and a member of the International Institute for Nanotechnology’s Steering Committee, is the corresponding author of the investigation, in collaboration with University of Maine professor Dean Astumian, a theorist in the department of physics and astronomy, and Omar Farha, an expert in MOF chemistry and a professor of chemistry at Northwestern. Liang Feng and Yunyan Qiu, postdoctoral fellows in Stoddart’s lab, are co-first authors of the paper.
“There’s good reason to believe that the concept of mechanisorption will command textbook attention one day,” Stoddart said. “If chemists can work out how mechanisorption can be incorporated in active structures, the storage of gases like hydrogen, carbon dioxide and methane will enter a whole new world and become a different ball game altogether.”
The research illustrates the synergy that results from combining theory with experiment. The idea of a pumping cassette arose from Astumian’s consideration of the effects of oscillating electric fields on membrane-bound enzymes. (A pumping cassette can be likened to a “valley” whose “floor” can be moved up and down surrounded by two “mountain passes” whose heights can be raised and lowered such that the molecules are obliged to move in one direction.) This molecular contraption was synthetically implemented in Stoddart’s laboratory using rotaxanes — long dumbbell-shaped molecules — terminated on one or both ends with a recognition site for rings surrounded by two groups to provide kinetic barriers between the bulk where the rings are swimming around in solution and polymer chains where the rings are collected one at a time following each redox cycle. Importantly, these barrier-forming groups can be designed to respond differently to changes in their environment. These pumping cassettes can be incorporated onto many types of polymer chains, giving rise to numerous possible applications.
Mechanisorption has important implications for storage and controlled release of many different molecules. This work focuses on recruitment of ring molecules to surfaces, but it is anticipated that these rings can be functionalized to bring many different types of molecules at high concentration to surfaces.
“The mechanisorption mechanism bears some features in common with spray cans,” Stoddart said, “where different materials are stored at high pressure and then released by pressing a trigger. The mechanisorbed substances, however, remain in mechanical equilibrium even while being packaged far from thermodynamic equilibrium. The mechanism of triggered release involves only diffusion, a process which, while seemingly slow from a macroscopic perspective, is remarkably fast in these systems.”
Astumian at University of Maine points out that the research is also important for understanding one of the deepest questions in chemistry. “What are the principles by which simple matter becomes complex?” he said. “A key point is that, while thermodynamics determines the most likely structures near equilibrium, kinetics plays the dominant role in selecting structures when far-from-equilibrium.”
In the 1930s, Irving Langmuir and John Lennard-Jones observed that adsorbates interact with surfaces through van der Waals interactions (physisorption) and/or electronic interactions (chemisorption). Adsorption is generally considered to be a passive process in which the adsorbate moves from a high to a low concentration area, so the concentration of surface adsorbate always changes towards the equilibrium direction. In the Northwestern study, however, the researchers demonstrate that active adsorption can be achieved using artificial molecular machines.
“The potential utility of mechanisorption in technology, such as chemical capacitors, will provide a completely new way to store and manipulate energy, information and matter on surfaces that have never been imagined before,” co-first author Feng said. “The advent of the mechanical bond is sending major ripples through both chemistry and materials science. Given a little more time, the general area of sorption will witness a profound change after close on a century during which period physisorption and chemisorption have dominated surface and interfacial science.”
Co-first author Qiu added, “This research is the first example of utilizing artificial molecular pumps to recruit and adsorb molecules actively onto solid surfaces and opens the door to operating artificial molecular machines on the surfaces of a range of functional materials, ranging from zeolites and metal oxides to polymer networks and micellar nanoparticles.”
Experts familiar with the work but not involved in the study noted the significance of the research and its potential applications.
“The extraction of chemicals from solution into and onto solids and surfaces underpins the sequestering of waste and pollutants, the recovery of precious metals, heterogeneous catalysis, many forms of chemical and biological analysis and separation science, and numerous other technologies,” said David Leigh, Royal Society Research Professor at the University of Manchester in the United Kingdom.
“Until now there has been no way to actively drive such processes, but the use of molecular machinery changes that, through a mechanism the Northwestern team term ‘mechanisorption,’” he said. “Miniaturization has driven advances in technology through the ages, and using machines the size of molecules — molecular nanotechnology — to power adsorption will surely continue that trend.”
Jonathan Sessler, holder of the Doherty-Welch Chair in Chemistry at the University of Texas at Austin, said of the research, “It is a game changer. It opens up a new chapter in the all-important and typically energy-intensive area of separations. The author team has shown for the first time that it is possible to use mechanically linked pumping strategies to concentrate highly charged species against a Coulombic gradient.
“The use of electrochemical methods to drive this chemical off-equilibrium process opens up the possibility of direct use of solar power to enable separations,” Sessler said. “Ultimately, this approach could allow for cost-effective capture, remediation and purification of key industrial targets, such as hydrocarbons, carbon dioxide and micropollutants. Shorter term, it is likely that counterion effects could be used to drive anion recognition, while the use of asymmetric and non-racemic threading entities might allow for chiral separations. The opportunities seem almost endless.”
Note: Fraser Stoddart, Liang Feng and Yunyan Qiu have a patent application lodged with Northwestern University based on this work.